■ COVID-19 Research in IBS Center for Cognition and Sociality
The new member of the coronavirus family, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China on December 2019. COVID-19 is the official name given by the World Health Organization (WHO) for the disease caused by SARS-CoV-2. As of October 1, 2020, SARS-CoV-2 has infected over 30 million people and caused more than 1 million deaths worldwide. SARS-CoV-2 is very contagious and mainly transmitted through respiratory droplets produced by coughing and sneezing, or possibly through surface contaminated by people coughing or sneezing on them. More critically, there have been reports about the possibility of this virus to transmit even before a virus-carrying person to show symptoms. Therefore, a low-cost, easy-access protocol for early detection of this virus while presymptomatic or asymptomatic is needed. In addition, there is an urgent need to develop quarantine material that is harmless to the human body and treatments for COVID-19. Because we (Center for Cognition and Sociality) have been using viruses as materials for our research for over 15 years and has operated a virus facility (https://www.ibs.re.kr/virusfacility/), we've had interest in SARS-CoV-2 virus from the early stage of COVID-19 outbreak and been continuing research on the following three topics.
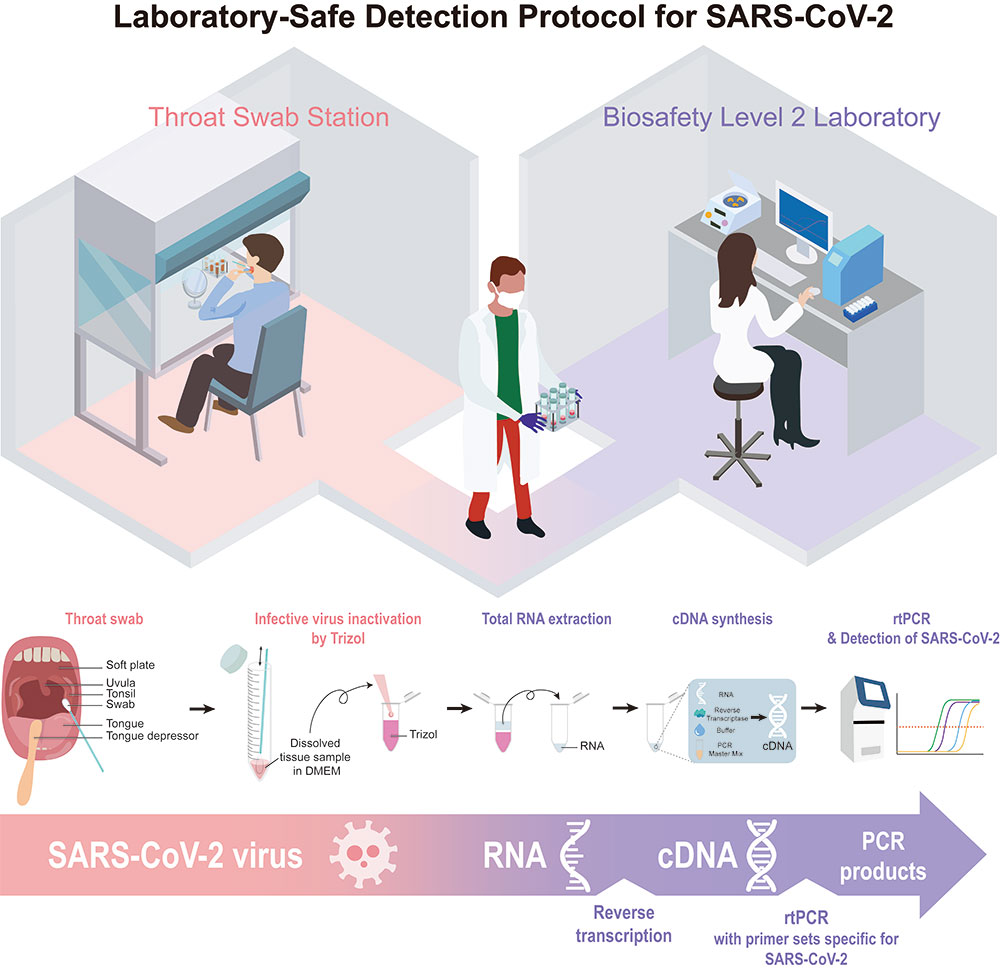
We have established a real-time reverse-transcription PCR (rtPCR)-based assay protocol composed of easy specimen self-collection from a subject via pharyngeal swab, Trizol-based RNA purification, and SYBR Green-based rtPCR. It is a cost-effective, quick-and-easy method for early detection of SARS-CoV-2 at any conventional Biosafety Level II laboratories that are equipped with a rtPCR machine.
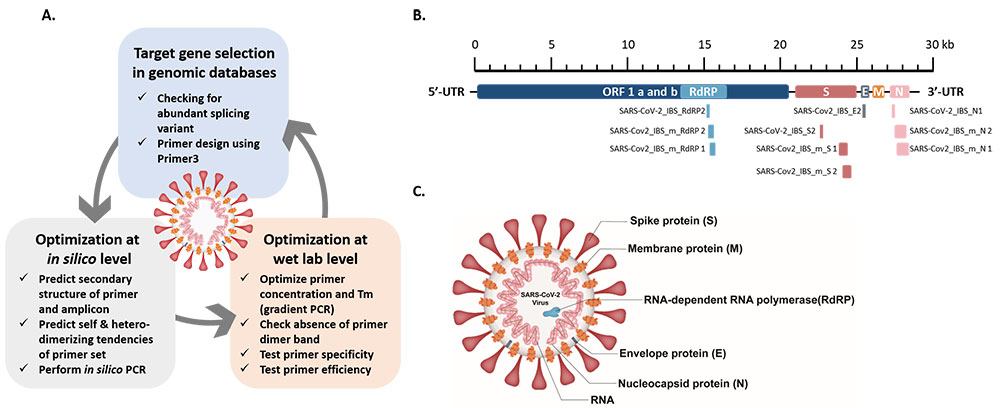
To reduce false-positive results we encountered in the previous study, We propose three-step guidelines for the design and optimization of specific primer sets. The three steps include (1) the selection of primer sets for target genes (RdRP, N, E, and S) in the genome of interest (SARS-CoV-2), (2) the in silico validation of primer and amplicon sequences, and (3) the optimization of PCR conditions (i.e., primer concentrations and annealing temperatures) for specific hybridization between the primers and target genes, and the elimination of spurious primer dimers. This newly optimized protocol should be helpful for the large-scale, high-fidelity screening of asymptomatic people, even without any high-specification equipment, for the further prevention of transmission, and to achieve early intervention and treatment for the rapidly propagating virus.
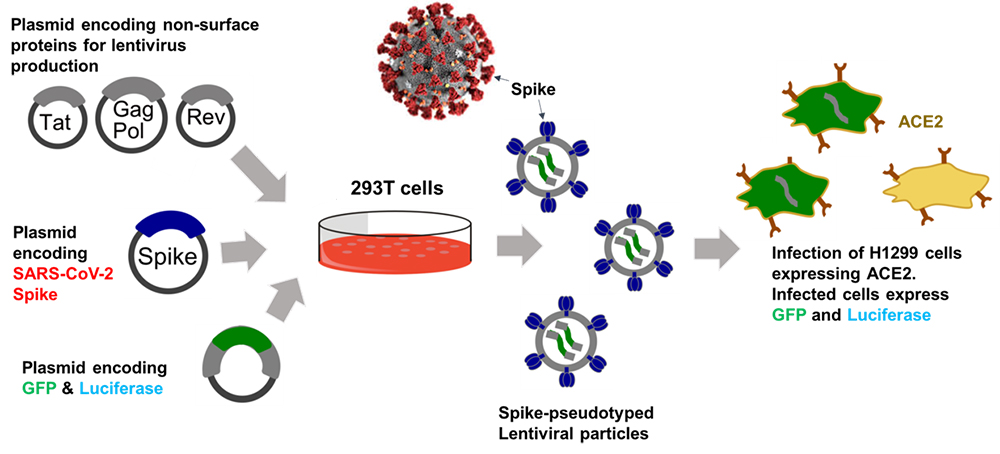
To study host cell infection of SARS-CoV-2, we constructed a replication-defective recombinant lentivirus pseudotyped with SARS-CoV-2 spike. The basic strategy involves the transfection of HEK293T cells with a lentiviral backbone plasmid encoding EmGFP and firely luciferase protein, lentiviral packaging plasmid, and a plasmid expressing SARS-CoV-2 spike. The spike protein of SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) on host cells.
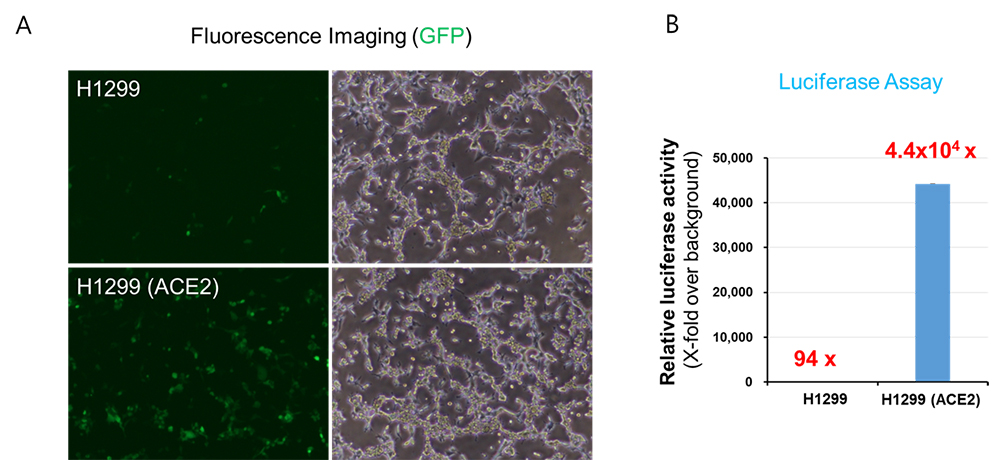
(A, B) Parental H1299 cells or H1299 cells stably expressing ACE2 were transduced with SARS-CoV-2 spike pseudotyped lentiviral particles containing GFP and firefly luciferase gene. At 2 days post-transduction, images were acquired using a fluorescence microscope (A) and transduction efficiency was determined by measuring luciferase activity in cell lysates. The activity obtained from untransduced cells were used for normalization (B).
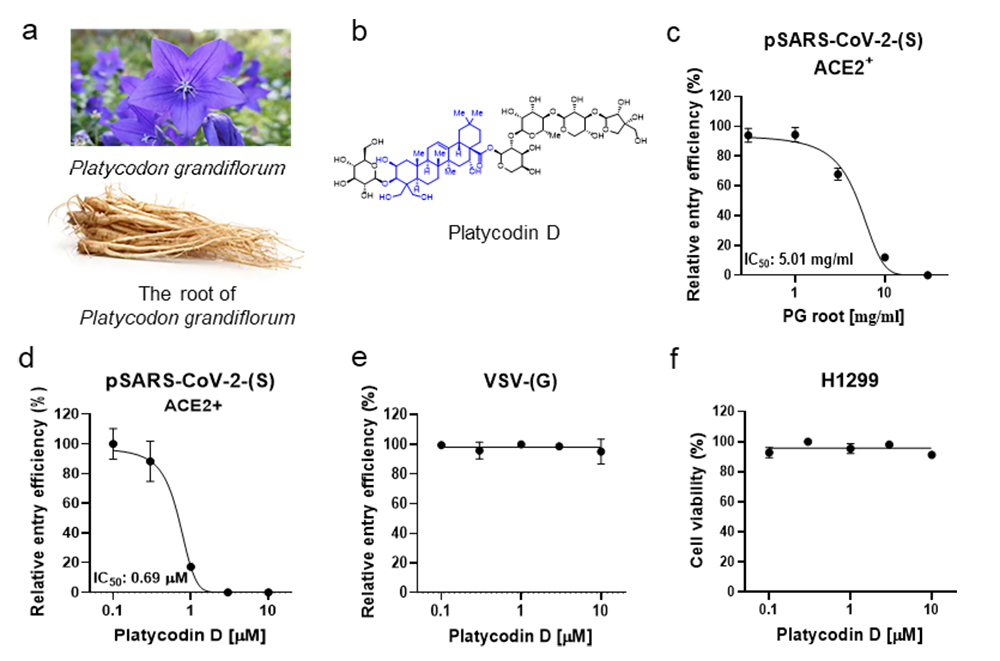
Herbal medicines and their derived natural products have drawn much attention to treat COVID-19. Among thousands of Korean traditional herbal medicines, we have focused on the root of Platycodon grandiflorum (PG) (a). The Dongui Bogam reports that PG can be used to treat patients with disorders in respiratory tracts and the lung, the major target sites of SARS-CoV-2 Several studies have demonstrated that the root of PG is enriched with platycodin D (PD) (b). In ACE2+ H1299 cells, we found that 1-hour treatment with PG and PD effectively reduced pSARS-CoV-2 entry in a dose-dependent manner with a half-maximal inhibitory concentration (IC50) of 5.01 mg/ml (Fig. 1c) and 0.69 μM (d), respectively. In contrast, PD did not block the entry of the control lentiviral particles driven by the glycoprotein (G proteins) of the vesicular stomatitis virus (VSV) (e). PD showed no cytotoxic effect on H1299 per se at the tested concentrations (f). These results indicate that the inhibitory effect of PD on virus-entry requires both S protein of SARS-CoV-2 and ACE2 in the host cells.
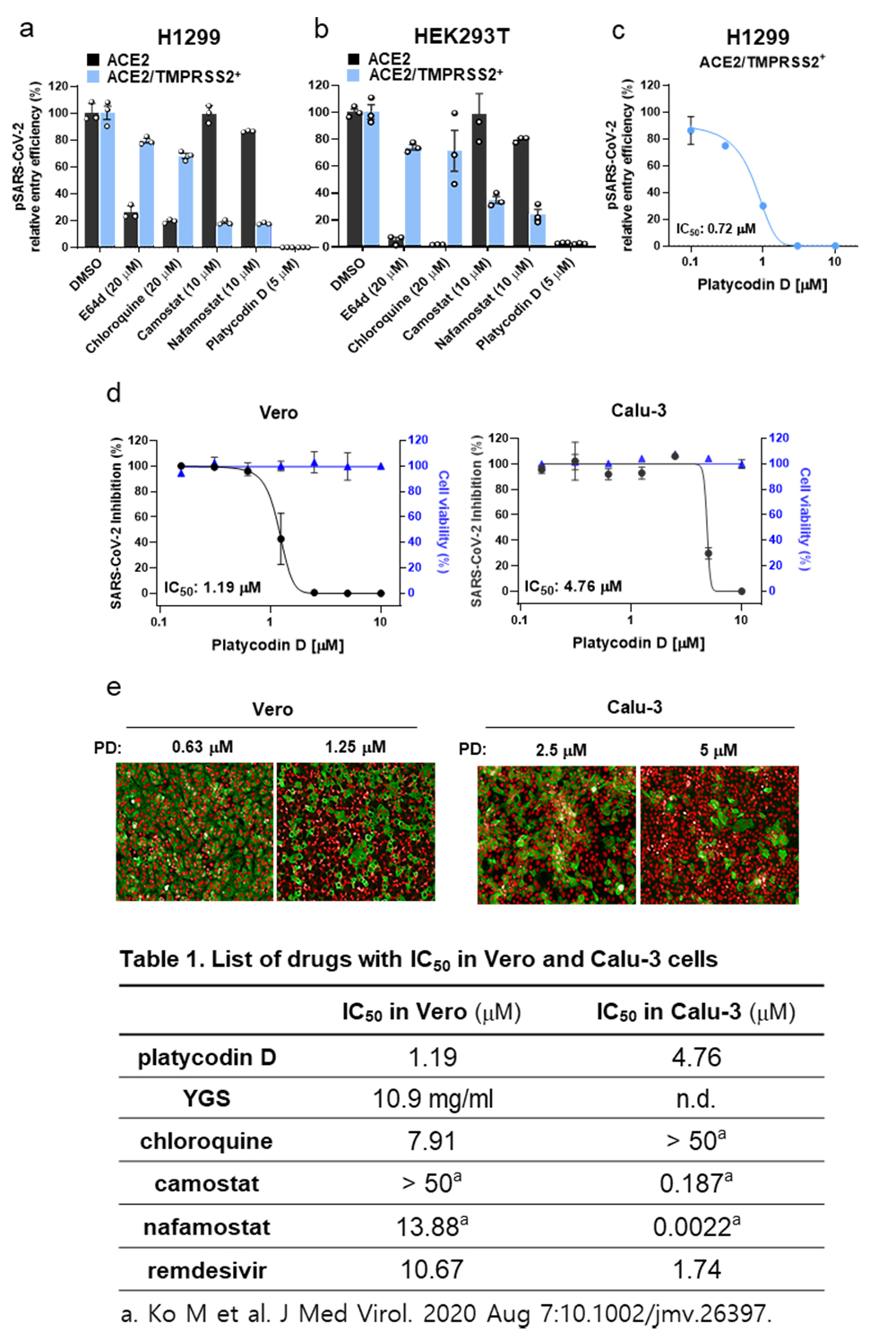
To determine which one of the two entry pathways is the target of PD, we prepared additional cell lines, H1299 and HEK293T, overexpressing both ACE2 and TMPRSS2 (ACE2/TMPRSS2+) and compared the inhibitory effect of various drugs with ACE2+. We found that E64d and chloroquine, inhibitors of lysosomal cathepsins, effectively blocked pSARS-CoV-2 entry only in ACE2+, but not in ACE2/TMPRSS2+, whereas camostat and nafamostat, inhibitors of TMPRSS2, effectively blocked pSARS-CoV-2 entry in ACE2/TMPRSS2+, but not in ACE2+ (a, b). In contrast, 5 M PD completely inhibited pSARS-CoV-2 entry in both ACE2/TMPRSS2+ and ACE2+. (a, b). The actual potency of PD for inhibition of pSARS-CoV-2 entry in terms of IC50’s in ACE2/TMPRSS2+ was determined to be 0.72 M (c). These results suggest that PD targets any one of the events that is common to both entry pathways. we evaluated the antiviral activity of PD on the authentic SARS-CoV-2 viruses obtained from the Korea Centers for Disease Control and Prevention. We found that PD caused a significant reduction in SARS-CoV-2 infection in both TMPRSS2-negative Vero and TMPRSS2-postive Calu-3 with IC50’s of 1.19 M and 4.76 M, respectively (d, e). Unlike other previously reported drugs (Table 1), PD shows the unique property of equal high potency against SARS-CoV-2 infection in both TMPRSS-negative Vero and TMPRSS-positive Calu-3 cells. This is in marked contrast to other drugs, which show only one-sided potency in either TMPRSS-negative Vero (chloroquine) or TMPRSS-positive Calu-3 (camostat, nafamostat).
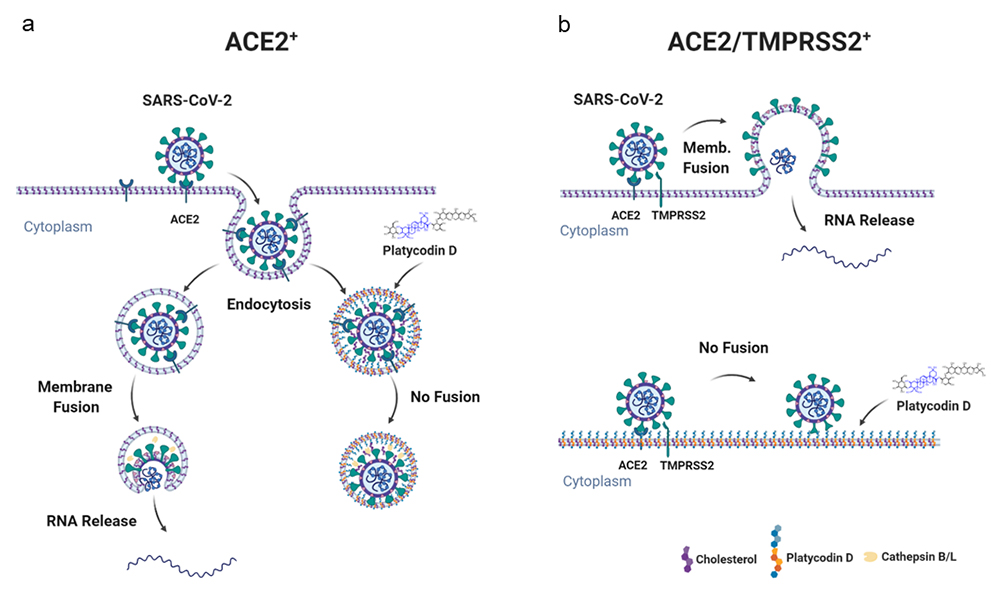
Taken together, these results indicate that PD has an important advantage over other known drugs in that it can potently prevent SARS-CoV-2 infection by inhibiting both lysosome- and TMPRSS2-driven SARS-CoV-2 entry pathways.