mainmenu
Cognitive Glioscience Group
Cognitive Glioscience group has contributed to the field of gliotransmission by creating several seminal publications on the channel-mediated gammaAminobutyric acid (GABA) and glutamate release from astrocytes. They later identified the biosynthetic pathway for astrocyte GABA and found monoamine oxidase B to be the key enzyme for GABA production which raised the possibility that astrocytes can directly participate in cognitive processes via astrocytic GABA.
The group also found a connection with GABA and H2O2 from reactive astrocytes and impaired memory in mouse models of Alzheimer’s disease, leading them to propose astrocytic GABA- and H2O2-related pathways might be a diagnostic tool, biomarker, and therapeutic target for both neurological diseases Alzheimer’s and Parkinson’s. The research is notable as it revealed that astrocytes, like neurons, play a significant role in cognitive processes. The findings also resulted in a technology transfer to Neurobiogen which will be prepared for a phase I clinical trial in 2022.
Thus, the group continues to investigate the cognitive functions of 1) GABA synthesis and release from glia, 2) molecular mechanisms of glutamate and d-serine release from glia, 3) astrocytic volume transient and brain plasticity, and 4) reactive astrogliosis and neurodegeneration.
- 1. GABA synthesis and release from glia
- 2. Molecular mechanisms of glutamate and d-serine release from glia
- 3. Astrocytic volume transient and brain plasticity
- 4. Reactive astrogliosis and neurodegeneration
Astrocyte, Reactive astrocyte, Astrocyte neuron interaction, GABA, Glutmate, Dserine, ATP release, Metabolism, Synaptic transmission and plasticity, Memory, Alzheimer’s disease, Parkinson’s disease, Spinal cord injury, Depression, PTSD
- Astrocytic urea cycle detoxifies Aβ-derived ammonia while impairing memory in Alzheimer’s Disease, Cell Metabolism, 2022
- Astrocytes Render Memory Flexible by Releasing D-Serine and Regulating NMDA Receptor Tone in the Hippocampus, Biol Psychiatry, 2021
- Opto-vTrap, an optogenetic trap for reversible inhibition of vesicular release, synaptic transmission, and behavior, Neuron, 2021
- A nonsense TMEM43 variant leads to disruption of connexin-linked function and autosomal dominant auditory neuropathy spectrum disorder, Proc Natl Acad Sci USA, 2021
- Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2-production, Nature Neurosci, 2020
- Astrocytes Control Sensory Acuity via Tonic Inhibition in the Thalamus, Neuron, 2020
- Aberrant Tonic Inhibition of Dopaminergic Neuronal Activity Causes Motor Symptoms in Animal Models of Parkinson’s Disease, Curr Biol, 2020
- 2022 Managing Director, Institute for life Sciences, IBS
- 2019 Director, Center for Cognition and Sociality, IBS
- 2017 Presidential Medal of Honor, Korea Science & Technology Development
- 2016 The Kyung Ahm Prize in Arts & Sciences
- 2014 The Korean Academy of Science and Technology, FILA Basic Science Award
- 2014 Jang Jin Award, Korean Society for Brain and Neuroscience
- 2013 Star Professor Award, The University of Science & Technology
- 2011 Scientist of the Year Award, KIST
- 2010 Outstanding Researcher Award, Prime Minister of Korea
- 2010 Scientist of the Month Award, Ministry of Science & Technology, Korea
- 2010 Scientist of the Month Award, KIST
- 2009 Outstanding Project Award, Outstanding Researcher Award, KIST
- 2009 Outstanding Teacher Award, The University of Science & Technology(UST)
- 2000, 2003 Outstanding Researcher Award, Association of Korean Neuroscientists(AKN)

Director C. Justin Lee
that regulate the brain functions
- Unraveling the mechanism of synthesis and release of gliotransmitters in astrocyte
- Understanding the mechanism of astrocyte-mediated brain plasticity
- Developing drug candidates targeting astrocyte for AD and other neurodegenerativ e diseases
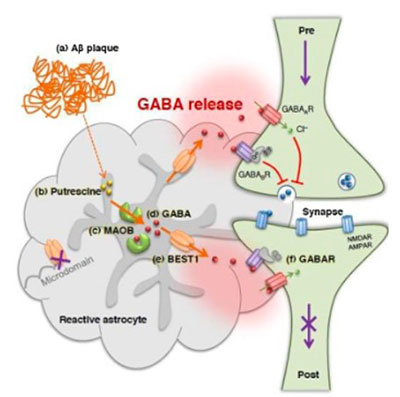
Our group has contributed to the field of gliotransmission (release and function of transmitters from glia) with seminal publications on the channel-mediated GABA and glutamate release from astrocytes. We demonstrated, for the first time, the channel-mediated release mechanism of the major inhibitory transmitter GABA from astrocytes in cerebellum involving Best1 channel and further demonstrated that the source of tonic inhibition in cerebellum is astrocytic GABA. We subsequently identified the biosynthetic pathway for GABA in astrocyte to be the putrescine degradation pathway leading to GABA production, in which the monoamine oxidase B (MAO-B) is the key enzyme for GABA production.
This series of studies implicates that astrocytes by tonically releasing the major inhibitory transmitter GABA, can exert strong inhibitory drive to the brain activity, raising a profound possibility that astrocytes can directly participate in cognitive processes via astrocytic GABA. Utilizing these newly identified molecular targets, Best1 and MAO-B, we further demonstrated that tonic GABA inhibition in cerebellum is critical for motor coordination.
In parallel, we have revealed the pathological role of astrocytic GABA, especially in Alzheimer’s disease (AD) and Parkinson’s disease (PD). We provide compelling pieces of evidence that hippocampal or SNpc astrocytes aberrantly produce GABA via MAO-B and release through Best1 channels in AD or PD, respectively. The aberrant GABA from astrocytes inhibits neighboring neuronal activity to impair memory in AD or to cause motor deficit in PD. We further propose that the astrocytic GABA could be an optimal biomarker, diagnostic tool, and therapeutic target for AD and PD. We continue to investigate the role of aberrant GABA from astrocytes in white matter stroke, recovery after spinal cord injury, obesity, and epilepsy.
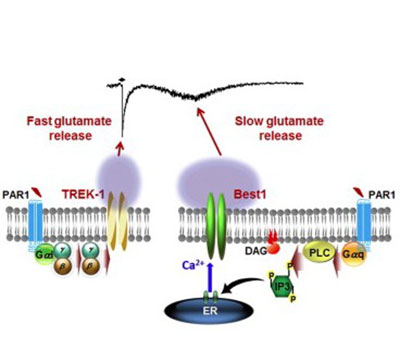
In addition to GABA, we have investigated the molecular mechanism of glutamate release from astrocytes because glutamate has been known to be released from astrocytes but the release mechanism has been controversial. Glutamate is the major excitatory transmitter that enhances neuronal excitability.
Through an intense investigation, we have clearly demonstrated that glutamate is released in two models: fast mode through TREK-1 containing K2P channel and in slow mode through Best1 channel in hippocampal astrocytes. We have subsequently reported that Best1-mediated glutamate release contributes to receptor mediated synaptic plasticity in hippocampus upon activation of PAR1 (a Gq-coupled GPCR). Through this series of studies, we have demonstrated that astrocytic glutamate and GABA are the key modulator for excitation-inhibition balance (E/I balance) in the brain, which is mainly dependent on the levels of glutamate and GABA. In addition to glutamate, we found that Best1 is also capable of releasing d-serine, which can act as a co-agonist of NMDA receptors to participate in synaptic plasticity. We are actively investigating the possibility of astrocytes as the potential therapeutic target for various brain disorders such as schizophrenia, autism, ADHD, epilepsy, depression, and etc, which are known to be caused by impaired E/I balance.
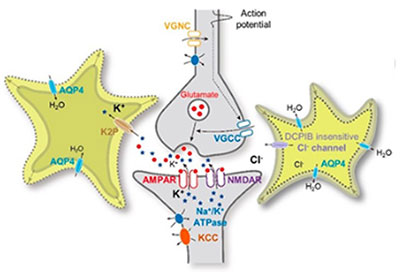
We have also contributed to the field of glioscience by identifying and characterizing several astrocytic ion channels. We identified that astrocytic two-pore potassium channel K2P, which is responsible for passive conductance with a subunit composition of a heterodimer of TWIK-1 and TREK-1. We further proposed the heterodimer of TWIK-1 and TREK-1 as the potential therapeutic target of epilepsy, depression, and anxiety disorders caused by dysregulation of potassium ion concentration. Next, we found the identity of astrocytic volume-regulated anion channel (VRAC), which is tweety-homolog (Ttyh).
So far the identity of VRAC has been proposed to be LRRC8. However, we firstly demonstrated that the pore-forming subunit of VRAC in astrocytes is Ttyh, but not LRRC8. More importantly, we have demonstrated for the first time that astrocytic volume change through aquaporin-4 water channel is critical for synaptic plasticity and established the novel concept of volume plasticity in both mouse and human. We have demonstrated that this astrocytic volume change is critical for spatial memory in mouse and language-associated learning and memory in human. This ground-breaking finding and novel concept of astrocytic volume will be the basis for a paradigm shift in brain plasticity from the traditional view of neuroplasticity to the new concept of glioplasticity and memory.
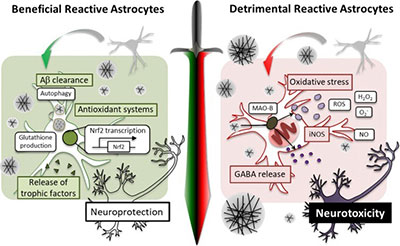
During the course of delineating the molecular and cellular mechanisms of gliotransmission, we have encountered the important concept of reactive gliosis, which is often referred to as the basis for neuroinflammation.
Although pathologic contributions of reactive astrocytes have been implicated in AD and other neurodegenerative diseases, their in vivo functions have remained elusive due to the lack of an appropriate experimental model. We have recently developed an astrocyte-specific toxin receptor model, and found that the astrocytes, which have an active autophagy system, not only remained alive but selectively became reactive. Our study identifies the severe reactive astrocytes as a key determinant of neurodegeneration in AD. We anticipate that this novel mechanistic insights will be very useful for developing drug candidates for AD and other neurodegenerative diseases, such as PD, traumatic brain injury, stroke, and etc.
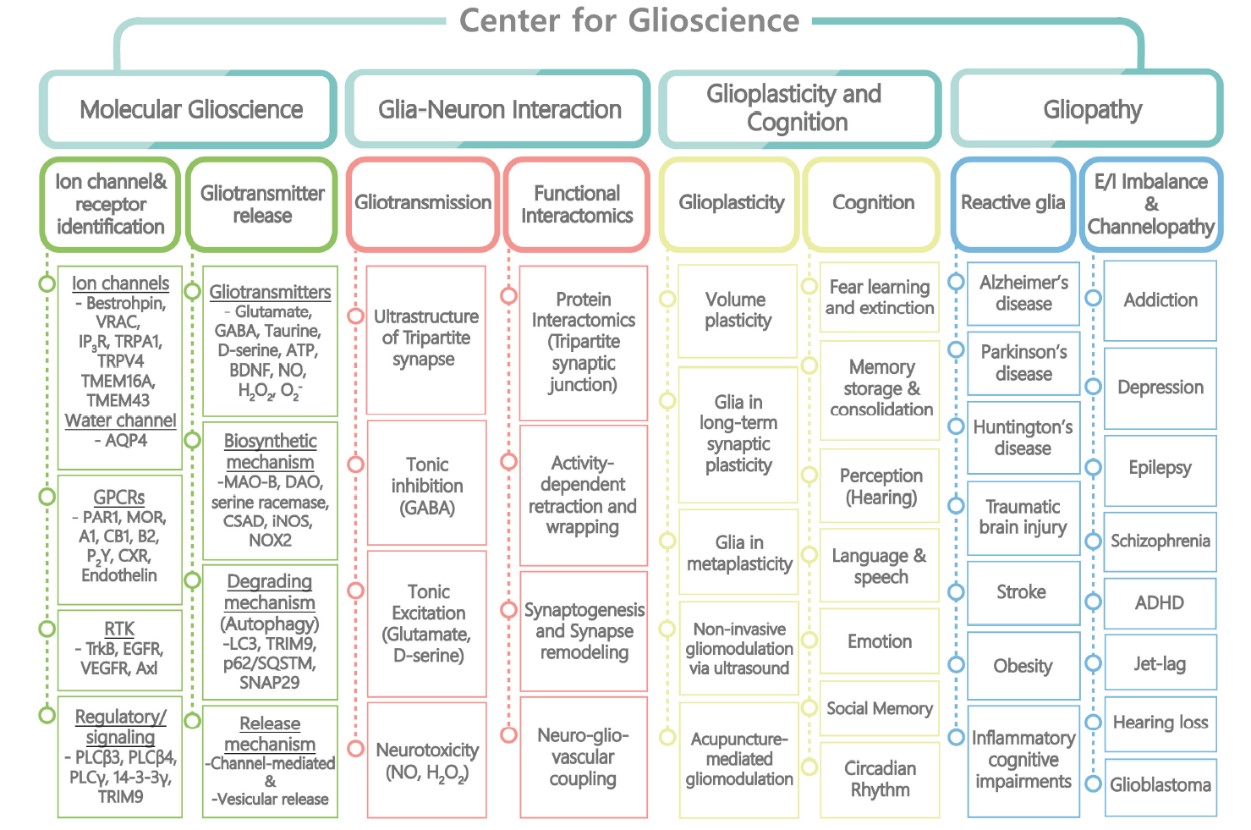
To realize the goals and visions of the Center for Cognitive Glioscience,
we will divide
the research areas based on the systemic level of research in terms of brain functions.
- A. Identification and characterization of novel glial ion channels, GPCRs and signaling and regulatory molecules.
- B. Molecular and cellular mechanisms of biosynthesis, degradation and release of gliotransmitters under physiological and pathological conditions
- A. Role of gliotransmitters in neuronal excitability, synaptic transmission, and neurotoxicity.
- B. Interaction between glia and neurons and vasculature via physical interactions or gliotransmitters.
- A. Role glia in brain structural plasticity as well as synaptic plasticity.
- B. Role of glia in various cognitive processes and behaviors.
- A. Pathophysiological role of reactive glia in rodents, non-human primate and humans.
- B. Role of gliotransmitters in E/I balance related diseases and channelopathies.

- Lab Homepage: https://www.ibs.re.kr/glia/
- Contact: Tel. +82-42-878-9155 (Lab)
- Intranet: https://www.ibs.re.kr/glia/
- Contact: PI: C. Justin Lee: Tel. +82-42-878-9150 (Office)/ 010-2825-7128, cjl@ibs.re.kr
We have a lab meeting on Mon and a journal club on Fri from 9:00 AM. During this meeting we will discuss lab business and present your own data collected in the past week. Attendance for each meeting is required for all members, please be prompt.
- 1) Lab meeting
- Organizer: Taeyung Kim
- 9:00 AM, every Monday, C239 seminar room
- Lab business: notes by Mingu Park
- Update for mouse status: Jae kyung Kwon
- Update for virus status: Solji Lee
- Update for administration: Dasom Kim
- Full work
- Presentation for one's recent main project
- One presenter at a time ordered by age (include Dr. Lee)
- Prepare as a Ph.D thesis defense for students and as a job talk for postdocs
- Personal progress
- Presentation for one's results in past 2 weeks and plans for next 2 weeks
- Each person is assigned for alternating weeks
- All files must be saved in the backup folder in ftp://203.247.189.127:4022 (Public NAS) before 9:00 a.m.
- 2) Journal Club
- Organizer: Taeyung Kim
- 9:00 AM, every Friday, C239, C241 seminar room
- Glance at journals: Yong min Park
- Update the interesting journal articles in current issue from assigned journals
- Email alert: Yong min Park
- Proposal and selection for the abstract of the most interesting paper for next week
- Journal club
- All participants should read the paper thoroughly before the journal club
- Presenter should prepare a ppt containing introduction, material and method, result (purpose, method, result and implication), and conclusion.
- Email sender and homepage update for the information of next journal club: Hyunji Kang
- All files must be saved in the backup folder in ftp://203.247.189.127:4022 (Public NAS) before 9:00 a.m.
Cleaning of your experimental environment is very important for your health as well as
quality of the experimental results. Therefore, we may ask you to clean up daily.
In every morning: Right after coming to lab, you may need to check the status of lab
bench.
- 1) Empty waste bottle.
- 2) Arrangement of all the stuffs in the right place such as pipettes.
- 3) Check floor and clean if any dirty stuffs.
- 4) If anything needs to be clean, clean up.
- 5) Monthly Cleaning:
- In every Monday of last week after lab meeting, we will clean all equipment and refrigerators. Because of limited space, at this time, you need to clean all the experimental stuffs nearby equipment and make a space in the refrigerators
- 6) Cell culture room manager
- Cell line: Minwoo Jang
- Primary culture: Youngmin Park
- Cell culture room cleaning every 2 weeks (rotation).
- Regular cleaning every 3 months.
- 7) Throw away of waste box by monthly rotation: Jae kyung Kwon makes a list.
- 8) Throw away of mouse carcass: Jae kyung Kwon.
Obtaining basic skills is essential for you to perform experiments properly.
- 1) Pipetting (training session by Eppendorf representative)
- 2) Properly calculating molarity and normality
- 3) Weighing and making stock solutions using volumetric flasks
- 4) Learn to use experimental machines and equipment
- Centrifuge (Moonsun Sa)
- Vortexer and stirrer (Yeonha Ju)
- pH-meter and osmo-meter (Mingu Park)
- Sonicator (Wuhyun Koh)
- Water-bath and heat block (Yeonha Ju)
- Clean bench and fume hood (Min-woo Jang)
- Perfusion clean bench and fume hood (Moonsun Sa)
- Incubator (Joungha Won, Wuhyun Koh, Jae Kwon)
- Deep freezer (Yongmin Park)
- Freezer (Each person in charge)
- Upright Microscopy (Joungha Won)
- Inverted Microscopy (Hyun ji Kang)
- PCR machine (Moonsun Sa)
- Electrophoresis gel runner (Hyun ji Kang)
- Electrophoresis gel doc (Moonsun Sa)
- Nanodrop (Joungha Won)
- Vibratome, slice patch puller and cell patch puller (Jea Kwon, Woojin Won, Min-woo Jang)
- Cryosection (Heeyoung An)
- Virus injector (Sunpil Kim)
- Electroporator (Joungha Won)
- Water purification system (Woojin Won)
- Heat block (Yongmin Park)
- 37℃ Incubator for molecular assay (Woojin Won)
- 5) Maintaining research consumables for experiments
- Ordering (Mingu park)
- Tip and tube (microtube and PCR tube) (Myungsun Park)
- 70% Ethanol (Yongmin Park)
- Media for cell culture and E.coli (Yongmin Park)
- Agarose gel and agar plate
- Stock for drug, antibody, enzyme, virus and DNA
- 6) Public duties
- Genotyping
- Tail prep (Jae kyung Kwon)
- Result should be reported by the next weaning time
- Each person is assigned of specific mouse lines
- Mouse maintaining: (Jae kyung Kwon)
- Each person should take training course on animal experimentation
- Each person should be fully informed about housing, weaning and ear-punching of mice
- Cell culture: primary astrocyte and neuron culture, mammalian cell lines (Myungsun Park, Yongmin Park)
- shRNA cloning & virus production: (Sol-ji Lee)
- Using essential experimental techniques including oligomer design, qPCR/RT-PCR, transformation, mini-prep, midi-prep, DNA sequencing, cell culture, transfection
- Receive order (cloning, virus production) from another institute
- Genotyping
- 7) Experimental techniques
- Primary astrocyte culture (Myungsun Park, Yongmin Park)
- Primary neuron culture (Yeonha Ju)
- Cell-line culture (Joungha Won)
- Calcium imaging (Sunpil Kim)
- FRET imaging (Jea Kwon)
- Two-photon imaging (Sunpil Kim)
- Cultured cell patch clamp recording (Min-woo Jang)
- Sniffer patch clamp recording (Wuhyun Koh)
- Pressure clamp recording (Hyun ji Kang)
- Slice patch clamp recording (Jea Kwon, Woojin Won, Joungha Won, Moonsun Sa)
- Slice field recording (Wuhyun Koh)
- Autopatch (Hyun ji Kang, Min-woo Jang)
- Immunohistochemistry (Heeyoung An)
- Behavior tests (Sunpil Kim)
- Microdialysis (Sunpil Kim)
- Optogenetic stimulation (Joungha Won, Woojin Won)
- Brain infusion with micropump (Sunpil Kim)
- Virus injection (Sunpil Kim)
- SNP analysis (Joungha Won)
- DNA cloning (Joungha Won)
- RNA work (Min-woo Jang)
- qRT-PCR (Joungha Won)
- Western blot (Min-woo Jang, Mingu Park, Taeyung Kim)
- DAO action assay (Wuhyun Koh)
- MAOB, H2O2 assay (Woojin Won)
- Confocal microscopy (Heeyoung An)
-
You can find more protocols from the backup folder of our lab
ftp://203.247.189.127:4022 (Public NAS)
- 8) Backing up data and presentation files (Wuhyun Koh)
- ftp://203.247.189.126:4022 (Private NAS)
- ftp://203.247.189.127:4022 (Public NAS)
- Each person needs username and password for access. (Password should be changed every 3 months)
- All raw data, analysis results, and presentations files must be backed up in these public drives
- 9) Lab home page maintenance (Wuhyun Koh)
- https://www.ibs.re.kr/glia/
- Update your personal info
Neural basis of perceptual awareness and statistical perception. We aim to better understand brain mechanisms of various perceptual, cognitive, and conscious processes by deploying multiple techniques ranging from behavioral methods such as psychophysics and eye tracking to brain imaging techniques such ECoG, EEG, and MRI. We also use machine learning techniques and bio-inspired computational modeling to probe the spatiotemporal pattern dynamics of brain imaging data and develop a mechanistic model of consciousness.
Cognitive neuroscience, Predictive coding, Statistical perception, Abstraction, General intelligence, Attention, Consciousness, Brain-imaging, Bio-inspired neural modeling
- Representational dynamics of sequential perceptual averaging, Journal of Neuroscience, 2022
- Induced astigmatism biases the orientation information represented in multivariate electroencephalogram activities, Human Brain Mapping, 2021
- Effect of spatiotemporally changing environment on serial dependence in ensemble representations, Biorxiv, 2021
- Ensemble representations reveal distinct neural coding of visual working memory, Nature Communications, 2019
- Attention to multiple objects facilitates their integration in prefrontal and parietal cortex, Journal of Neuroscience, 2017
- 2020-Present Associate professor, University of Science and Technology
- 2014-Present Research Fellow, IBS
- 2010-2014 Postdoc, Smith-Kettlewell Eye Research Institute, San Francisco, USA
- 2008-2010 Postdoc, Center for Neural Science, NYU, USA
- 2008 PhD in Brain, Behavior, and Cognition Program, Northwestern University, USA
Our laboratory's primary goal is to develop non-invasive neural modulation algorithms that reliably enhance brain function over the long term. To achieve this, we utilize electrophysiology and in vivo imaging to understand brain signal transmission and network interactions, aiming to modulate neuroplasticity non-invasively in both normal brain function and models of brain disorders and neurological conditions.
The core of our research focuses on non-invasive ultrasonic neuromodulation. Our objective is to develop innovative ultrasonic neuromodulation techniques that modulate brain activity without surgical intervention, thereby enhancing brain function safely and effectively.
We are developing new non-invasive ultrasonic neuromodulation technologies for clinical applications while also exploring alternative treatments for neurological and psychiatric disorders. To this end, we employ various electrophysiological and behavioral analysis techniques, including Whole-Cell Patch Configuration, Brainwave (EEG) Recording, High-Resolution Fluorescence Microscopy, Optogenetics, and Behavioral Modeling of Impaired Sensorimotor Gating.
Our research aims to thoroughly understand the neural circuits and synaptic plasticity involved in learning and memory. These creative and challenging projects are expected to significantly contribute to the growth of young researchers and expand our academic influence both domestically and internationally.
Cognitive and behavioral neuroscience, Cellular and molecular neurophysiology, Learning and memory
- Kim et al., Long-lasting Forms of Plasticity through Patterned Ultrasound-induced Brainwave Entrainment, Science Advances, 2024
- Phan et al., BDNF/TrkB Signaling Inhibition Suppresses Astrogliosis and Alleviates Mechanical Allodynia in a Partial Crush Injury Model, Experimental Neurobiology, 2023
- Lee et al., Ultrasonocoverslip: In-Vitro Platform for High-Throughput Assay of Cell Type-Specific Neuromodulation with Ultra-low-intensity Ultrasound Stimulation, Brain Stimulation, 2023
- Lee et al., Enhanced mitochondrial DNA editing in mice using nuclear-exported TALE-linked deaminases and nucleases, Genome Biology, 2022
- Lin et al., Homer1a Regulates Shank3 Expression and Underlies Behavioral Vulnerability to Stress in a Model of PhelanMcDermid Syndrome, Cell Reports, 2021
- Lee et al., Excitatory synapses and gap junctions cooperate to improve Pv neuronal burst firing and cortical social cognition in Shank2-mutant mice, Nature Communications, 2021
- Kim et al., Histone demethylase PHF2 activates CREB and promotes memory consolidation, EMBO Reports, 2019
- Kim et al., Association of mGluR-Dependent LTD of Excitatory Synapses with Endocannabinoid-Dependent LTD of Inhibitory Synapses Leads to EPSP to Spike Potentiation in CA1 Pyramidal Neurons. Journal of Neuroscience, 2019
- 2024-Present Adjunct Professor, IBS-SKKU Neuroscience program
- 2022-Present Adjunct Professor, IBS-UNIST Neuroscience program
- 2017-Present Professor, Basic Science, University of Science and Technology
- 2015-Present Research Fellow/Principal Investigator, IBS, Korea
- 2012-2015 Assistant Professor, Dept. Physiology, Jeju National University School of Medicine, Korea
- 2004-2012 Postdoctoral Fellow/Research Associate, Dept. Neuroscience, Johns Hopkins University School of Medicine, USA
- 2004 Ph.D. Dept. Physiology, Seoul National University College of Medicine, Korea
- 2014-2015 The distinguished Scientist Support program funded by Jeju National University
- 1. Developmental roles of astrocytes
- 2. Astrocytes in autism spectrum disorder (ASD)
- 3. Attachment formation and sociality development
- 4. Role of enteric glia in gut-brain axis
Astrocyte, Astrocyte Maturation, Astrocyte Morphogenesis, Autism Spectrum Disorder (ASD), Reactive Astrocyte, Astrocyte-Neuron Interaction, GABA, Attachment Formation, Synaptic Transmission and Plasticity, Memory, Enteric Glia, Postpartum Depression
- Tonic NMDAR Currents in the Brain: Regulation and Cognitive Functions. Biol. Psychiatry (2024)
- Diagnostic and therapeutic potential of tonic gamma‐aminobutyric acid from reactive astrocytes in brain diseases. Clin. Transl. Med. 14, (2024)
- In vivo magnetogenetics for cell-type-specific targeting and modulation of brain circuits. Nat. Nanotechnol. 1–11 (2024)
- GolpHCat (TMEM87A), a unique voltage-dependent cation channel in Golgi apparatus, contributes to Golgi-pH maintenance and hippocampus-dependent memory. Nat. Commun. 15, 5830 (2024)
- GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci. 1–17 (2023)
- Kv7/KCNQ potassium channels in cortical hyperexcitability and juvenile seizure-related death in Ank2-mutant mice. Nat. Commun. (2023)
- Astrocytes Render Memory Flexible by Releasing D-Serine and Regulating NMDA Receptor Tone in the Hippocampus. Biol Psychiat (2022)
- Astrocytes Control Sensory Acuity via Tonic Inhibition in the Thalamus. Neuron (2020).
- 2024 Research Fellow, Center for Cognition and Sociality, IBS
- 2022 Best poster award at the Annual Glia Conference of the Korean Society for Brain and Neural Science (KSBNS)
- 2021 Young Scientist Fellow (YSF), Center for Cognition and Sociality, IBS
- 2021 Researcher of the Year Award, IBS
- 2020 Best poster award at the Korean Society for Brain and Neural Science (KSBNS)
- 2018 Best oral presentation award at the Brain Science Institute (BSI), KIST
- Biophysical basis of information processing by neurons and neural circuits
- AI-based methods to analyze neuroscience data and build computational models
- Computational roles of physiological mechanisms
- Predictive computation by the cerebellum and other brain regions
Computational neuroscience, NeuroAI, Cerebellum, Sensorimotor learning, Biophysical neural modeling, Network dynamics
- Markanday, A.*, Hong, S.* et al., Multidimensional cerebellar computations for flexible kinematic control of movements. Nat. Commun., 14, 2548 (2023).
- Lindeman, S.*, Hong, S.*, Kros, L.* et al. Cerebellar Purkinje cells can differentially modulate coherence between sensory and motor cortex depending on region and behavior. Proc. Natl. Acad. Sci. USA 118, e2015292118 (2021).
- Han, D., De Schutter, E. & Hong, S. Lamina-specific neuronal properties promote robust, stable signal propagation in feedforward networks. Adv. Neural Inf. Process. Syst. 33, 3033–3044 (2020).
- Myung, J.*, Schmal, C.*, Hong S.* et al. The choroid plexus is an important circadian clock component. Nat. Commun. 9, 1062 (2018).
- Hong, S. et al. Multiplexed coding by cerebellar Purkinje neurons. eLife 5, e13810 (2016).
- Ratté, S., Hong, S., De Schutter, E. & Prescott, S. A. Impact of neuronal properties on network coding: roles of spike initiation dynamics and robust synchrony transfer. Neuron 78, 758-772 (2013).
- 2024- Research Fellow, Center for Cognition and Sociality, IBS.
- 2012-23 Group Leader, Computational Neuroscience Unit, Okinawa Institute of Science and Technology (OIST)
- 2007-12 PostDoc Researcher, Computational Neuroscience Unit, OIST
- 2004-07 Senior Fellow, Department of Physiology and Biophysics, University of Washington
- 2004 PhD in Theoretical High Energy Physics, University of Pennsylvania
 Center for Memory and Glioscience
Center for Memory and Glioscience








 Center for Memory and Glioscience
Center for Memory and Glioscience