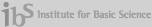mainmenu
Prof. Kim, Won Jong
Intelligent Nanomedicines for Therapy
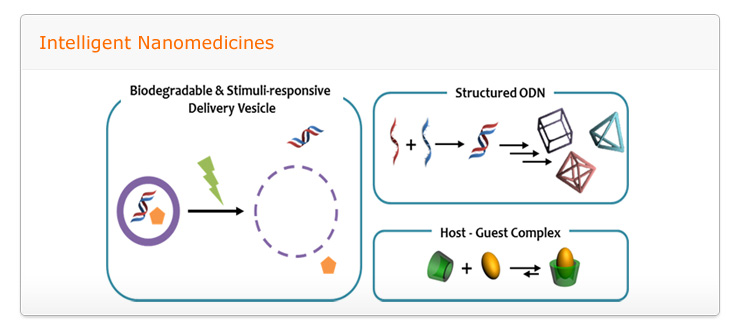
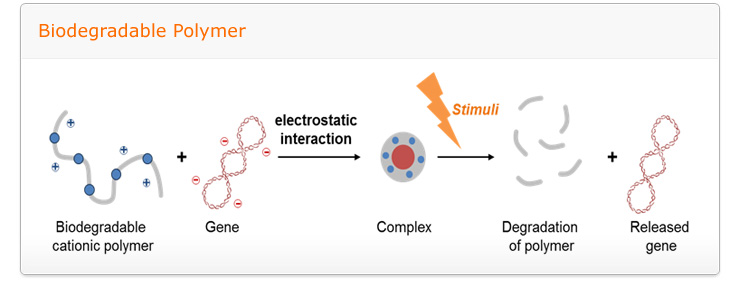
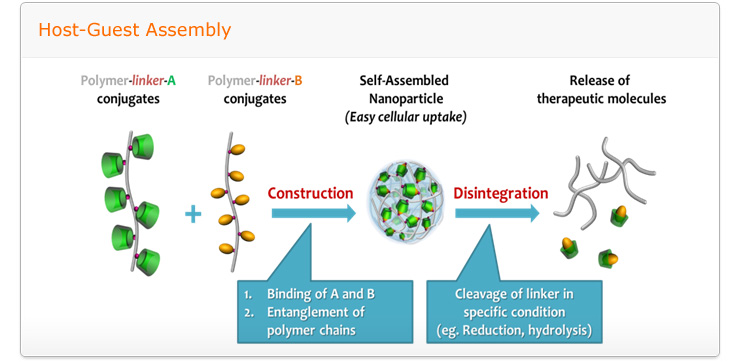
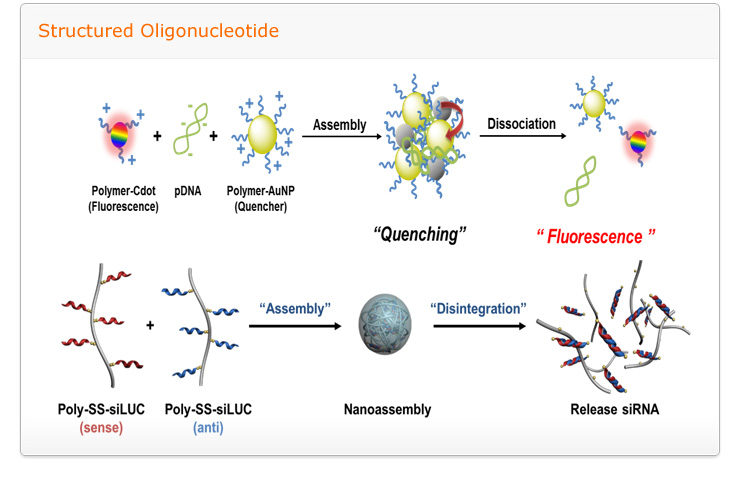
Goal: to develop of intelligent nanomedicine which made by self-assembled structure consist of cationic polymers, oligonucleotides, host-guest pairs and led stimuli-responsive, targeted drug/gene delivery with biocompatibility
A relentless scientific impetus to develop novel and more potent therapeutic agents which include small chemical drug molecules, proteins, and nucleic acids are invariably encountered with several impediments namely poor solubility in physiological condition, instability in circulation, inadequate tissue distribution and the efficacy of the therapies are either compromised to some extent or impaired greatly. Therefore to complement the drug development process, smart drug delivery systems has become one of the most intriguing aspects of this scientific pursuit. To take up the challenge to construct smart and efficient delivery systems, we will utilize self-assembly of integrated structures stemming from diverse molecular interactions. The self-assembly systems is expected to open up myriad of opportunities to modulate the extraordinary characteristics of drug delivery carriers through self-governing association of multiple components into programmed structures evading the adoption of intricate processes.
DNA Analysis Systems for Diagnosis
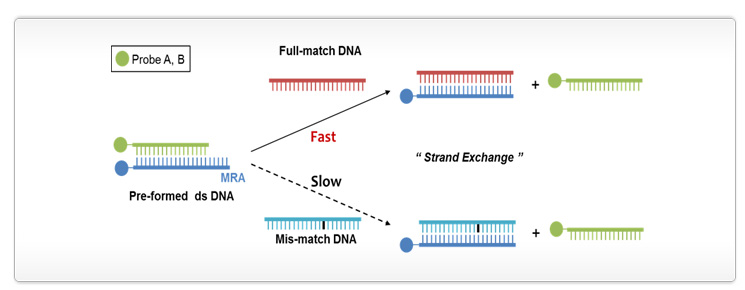
Goal: to develop a DNA analysis with accurate, rapid, and cost effective technologies based on a molecular recognition agent that consists of overhanging single-stranded DNA fully hybridizable with a target sequence using various nanomaterials
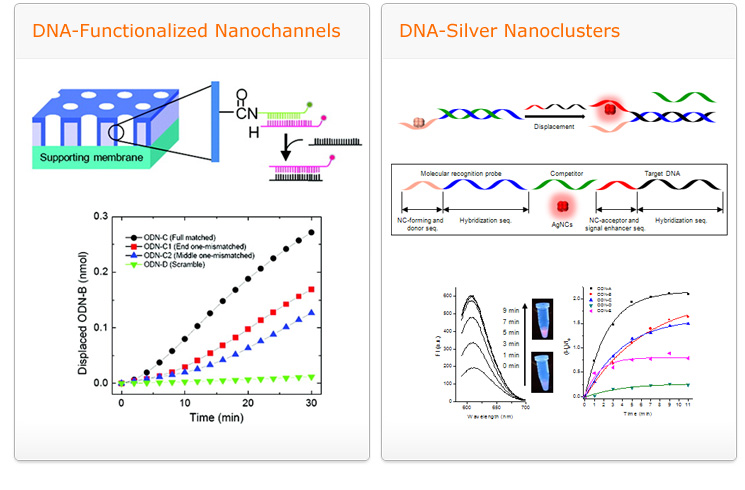
Single nucleotide polymorphisms (SNPs) are the most abundant forms of polymorphisms and contain a single nucleotide variation at a specific location in the human genome and consequently, links to phenotypic changes (alternations in protein structure and function). As SNP leads to the development of disease it could be served as genetic markers in many disease genetics and pharmacogenomic studies in order to prevent and treat human diseases as well as develop personalized medication. In our endeavor, we design accurate, rapid, and cost effective technologies for SNP analysis based on a molecular recognition agent (MRA). Compared to prevalent SNP detections the developed system works without the aid of enzyme substances and fluorophore-labeling. Furthermore, this system could be installed to nanostructures including nanochannels (kinetic-based resolution enhancement), gold nanoparticles (surface plasmon-based assay), ODN-templated silver nanoclusters (innate fluorescence-based assay), and could be combined to silver nanocluster-carbon dots (FRET-based assay) for better performance in SNP analysis. The potential of this proposed strategy could easily be translated into the real time monitoring of mRNAs in cellular processes due to the comparable size of nanomaterials and biomolecules.
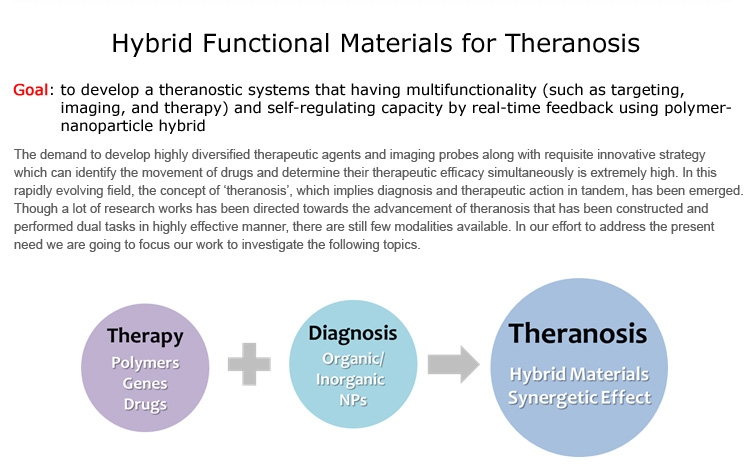
Hybrid Functional Materials for Theranosis
Goal: to develop a theranostic systems that having multifunctionality (such as targeting, imaging, and therapy) and self-regulating capacity by real-time feedback using polymer-nanoparticle hybrid
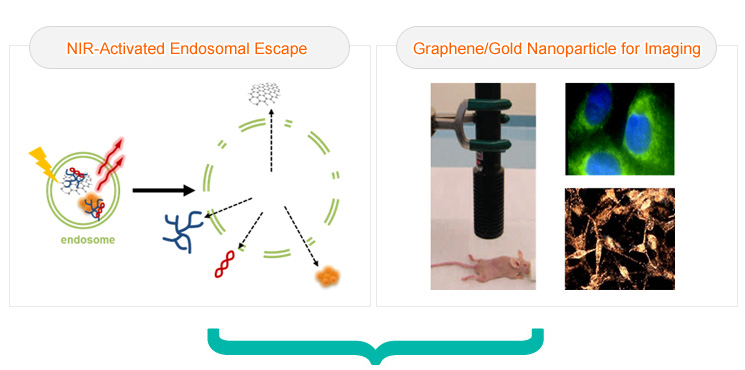
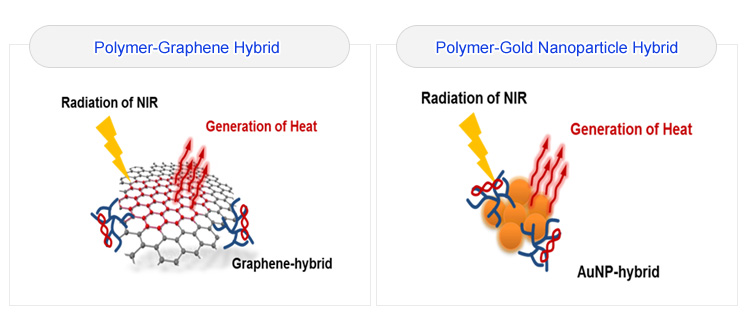
The demand to develop highly diversified therapeutic agents and imaging probes along with requisite innovative strategy which can identify the movement of drugs and determine their therapeutic efficacy simultaneously is extremely high. In this rapidly evolving field, the concept of ‘theranosis’, which implies diagnosis and therapeutic action in tandem, has been emerged. Though a lot of research works has been directed towards the advancement of theranosis that has been constructed and performed dual tasks in highly effective manner, there are still few modalities available. In our effort to address the present need we are going to focus our work to investigate the following topics.