Kwangseog Ahn
I. HCMV virology: Mechanism of Latency-Reactivation
Human cytomegalovirus (HCMV) is a ubiquitous virus with worldwide seroprevalence. Similar to other herpesviruses, HCMV establishes latency and persists throughout an individual's life. It remains the most common congenital infection and infectious complication in immunocompromised patients. Given the extensive and frequent occurrence of HCMV infections, as well as the significant socioeconomic burden they impose, the development of an HCMV vaccine has been a top priority for over 20 years. We are working to understand how host responds to HCMV during latency-reactivation, and how virus escape immune surveillance in latent-infected cells.
Q1. Deciphering the role of viral long non-coding RNAs in HCMV latency-reactivation.
HCMV lncRNAs are the most abundant transcripts in viral transcriptome through the entire life cycle, while their functions are largely unknown. We are focusing on how HCMV lncRNAs contribute to immune surveillance for establishing lifelong infection.
Q2. Regulation of HCMV latency-reactivation by the epitranscriptomic modification of viral RNAs.
Modifications on mRNA regulates diverse RNA biology from its decay to translation. We seek to define the role of epitranscriptomic modifications on HCMV RNA during HCMV latency and reactivation.
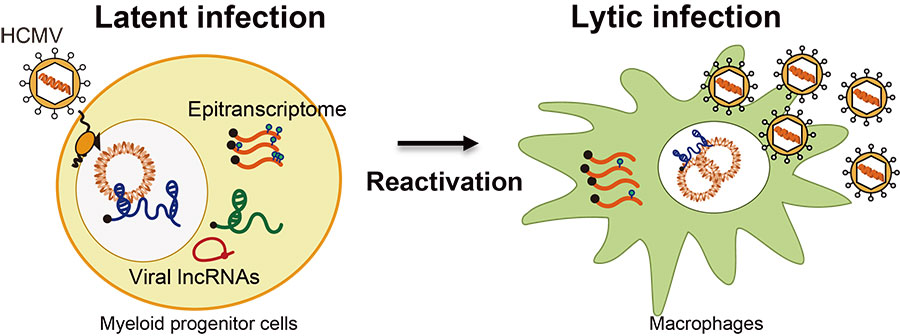
II. The interplay among retrotransposon, HCMV, and host cells
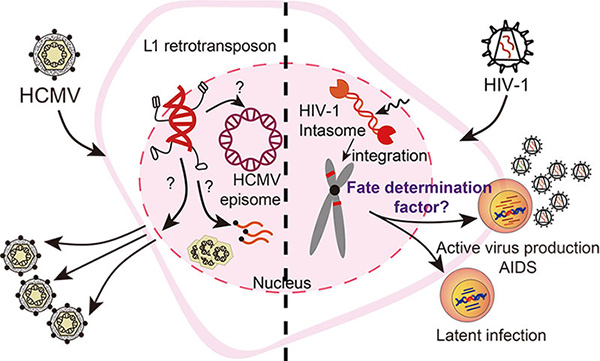
Q1. The interactions between L1 retrotransposon and HCMV
Long interspersed element-1 (L1) is currently an active autonomous retrotransposon, and constitutes ~17% of the human genome. The mobility of L1s contributed to a source of genetic variation, but also pose a threat to genome integrity. Both HCMV and L1s are closely associated with various human diseases. Our lab is exploring potential interactions among HCMV-retroelements-host and their impact on human diseases.
Q2. Molecular and cellular mechanisms of HIV-1 integration targeting
Retroviruses cause permanent infection in the host by integrating their reverse-transcribed viral genome into the host genome. Retroviral integration considerably impacts a wide range of biological phenomena, including the persistence of fatal human diseases and the shaping of metazoan evolution. The chromosomal landscape of HIV-1 integration plays a critical role in proviral gene expression, persistence of integrated proviruses, and prognosis of antiretroviral therapy. We are studying the mechanism by which HIV-1 select target sites for genomic integration.
 Center for RNA Research
Center for RNA Research